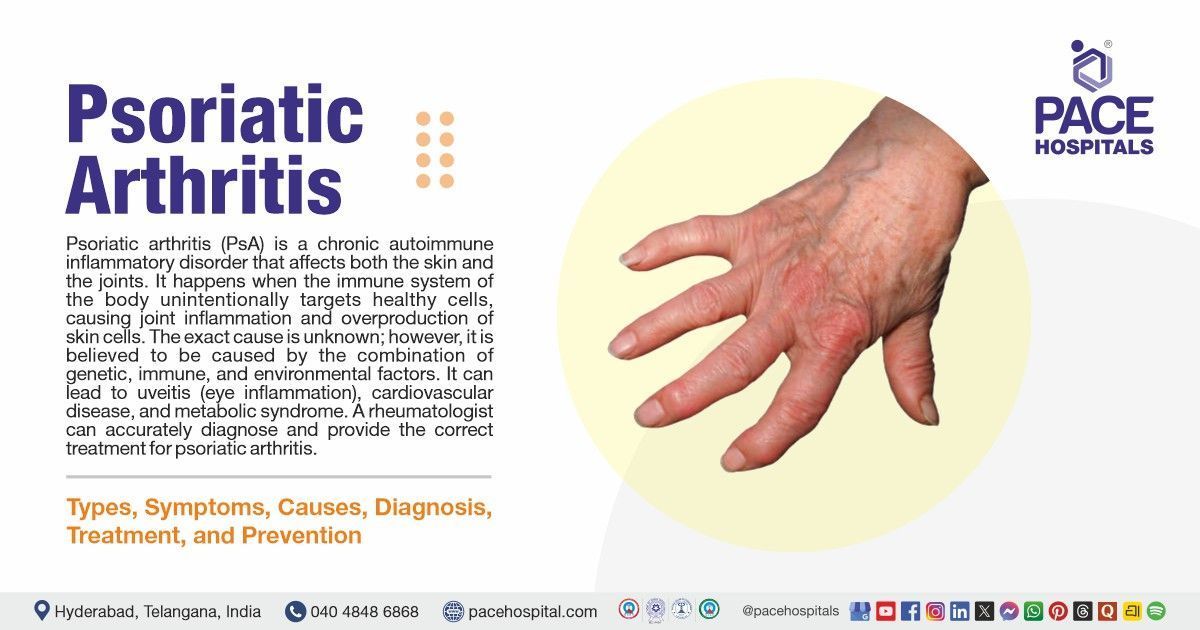
Sputnik V Vaccine - Efficacy, Doses, Side Effects and Price
COVID-19 Vaccine can help in protecting people and controlling the pandemic situation caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). Many scientist and countries are working towards development of efficacious vaccine.
The Sputnik V Vaccine (Gam-COVID-Vac) is one of three COVID-19 vaccine in the world with efficacy of over 90% and became the world’s first registered vaccine against coronavirus. More than 68 countries including India, UAE, Brazil, Hungary, South Korea, Argentina, Iran, Kenya, Mexico, China and Philippines are administering it to their citizens that will help all to fight against COVID-19. 40% of the global population or 300 crore (3 billion) people have access to Sputnik Vaccine.
The Sputnik V Vaccine has been developed by Gamaleya National Research Center for Epidemiology and Microbiology of the Ministry of Health of the Russian Federation, Moscow, Russia.
The global market Gam-COVID-Vac supplies will be produced by The Russian Direct Investment Fund (RDIF) through its international partners in India, South Korea, China, Brazil, and other countries.
What is the Sputnik V Vaccine and its mechanism of action?
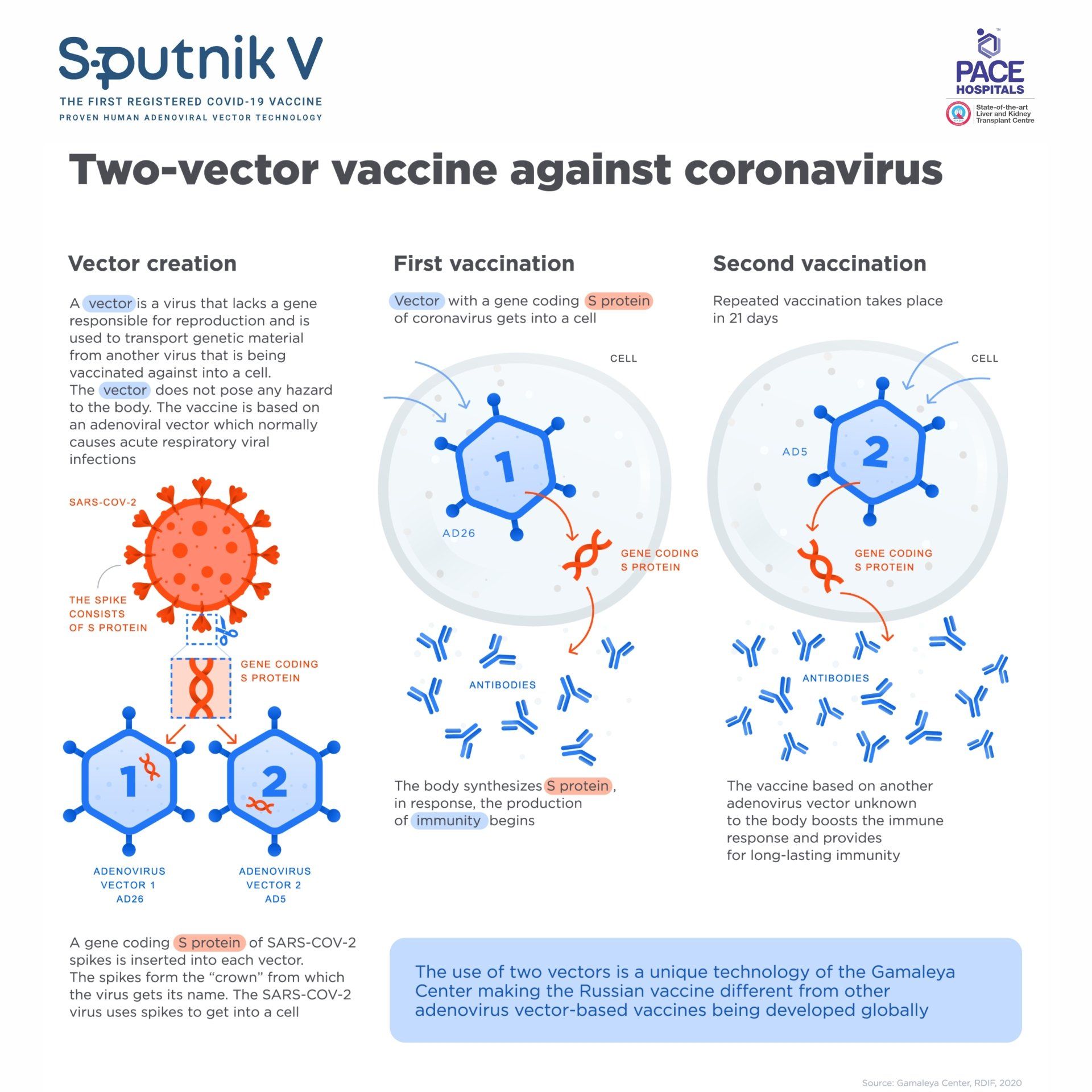
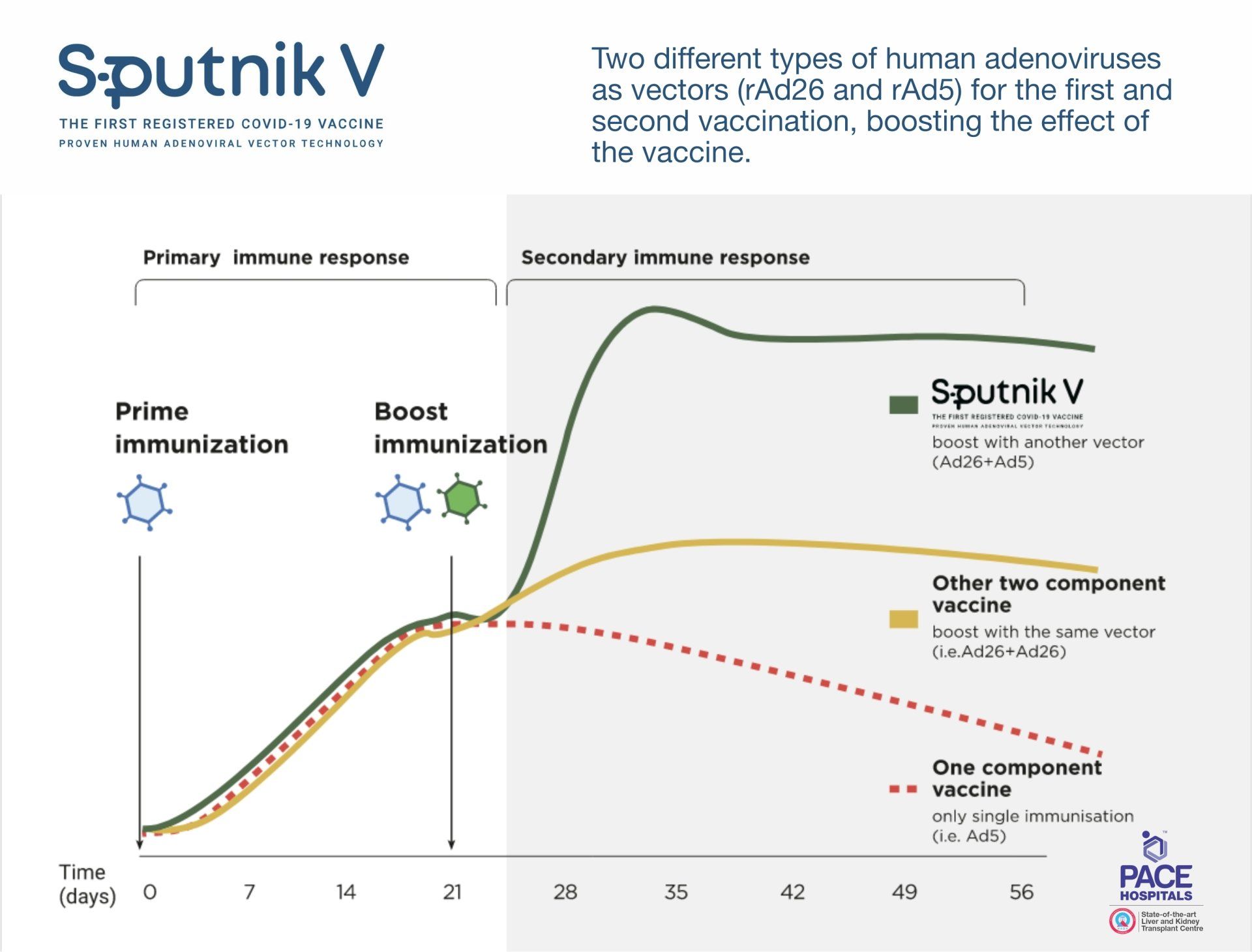
The Sputnik V (Gam-COVID-Vac) is based on safe and effective human adenovirus vector platform using two different adenoviral vectors - Adenovirus 26 (Ad26) and Adenovirus 5 (Ad5) as an expression of SARS-CoV-2 spike protein gene.
The use of two varying serotypes is a unique approach that provide long-lasting immunity and allows to boost the immune response. The carrier viruses are modified and cannot begin a productive infection; they enter cells, express the spike protein, and then stop. High sensitivity recorded that a few adenovirus genes were expressed, although at very low level.
Eventually, the vaccine-infected cells are destroyed by the very immunity they are designed to evoke. Recombinant adenoviruses (rAD) have been used widely as vaccine vectors due to their quality to accommodate large genetic payloads and, although unable to replicate, they trigger the intuitive immunity sensors sufficiently to make sure a robust immune system in nature.
A heterologous recombinant adenovirus approach is shared with the chimpanzee adenovirus (ChAdOx) vectored COVID-19 vaccine by Oxford/AstraZeneca, Adenovirus 26 (Ad26) vectored COVID-19 vaccine by the Johnson & Johnson, and the Adenovirus 5 (Ad5) vectored COVID-19 vaccine by CanSinoBIO-Beijing Institute of Biotechnology.
The Sputnik V Vaccine WHO approval
The Sputnik V Vaccine yet to receive approval for emergency use (EUL) from the World Health Organization (WHO) and European Medicines Agency (EMA). The WHO Emergency Use Listing Procedure (EUL) will initiate widespread distribution and make it eligible for COVAX (COVID-19 Vaccines Global Access) initiative that can provide equitable access to COVID-19 vaccine doses for Low-Income Countries.
In spite of not having WHO and EMA emergency use approval, the Sputnik V Vaccine is already registered in more than 70 countries simultaneously these countries have been started administering the vaccine to their people.
The Sputnik V Vaccine efficacy -
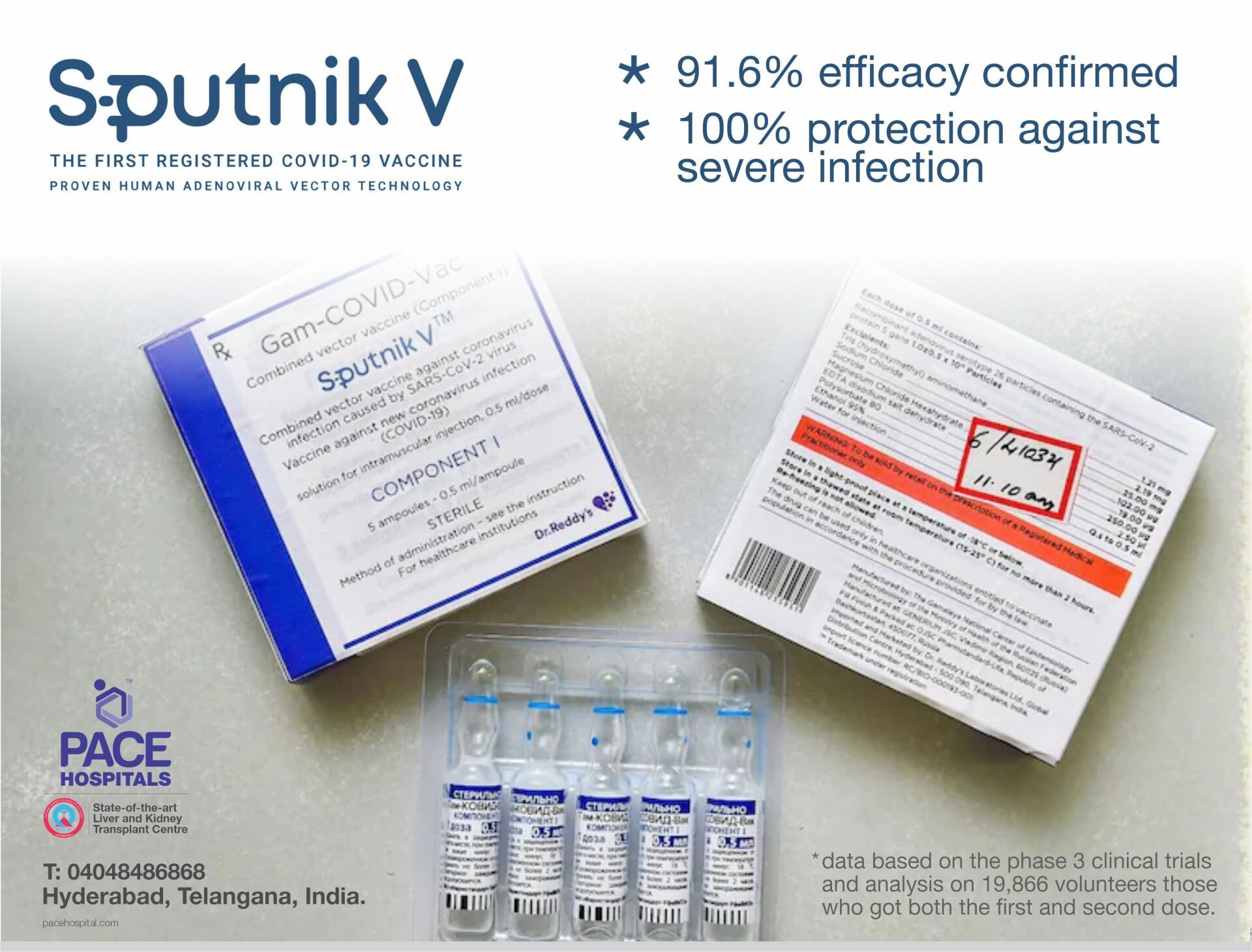
The Sputnik V Vaccine recorded 91.6% efficacy based on the clinical trials and analysis on 19,866 volunteers, those who got both the first and second dose. Efficacy was validated by internationally peer reviewed data published in The Lancet after phase 1 and phase 2 clinical trials.
As per the recent data based on the 3.8 million people vaccinated with two doses in Russia; Sputnik V COVID-19 vaccine shows 97.6% efficacy.
Also, United Arab Emirates Ministry of Health released data of 81,000 individuals who had got two doses of the vaccine, reported 97.8% efficacy in preventing symptomatic COVID-19 infection and 100% efficacy in preventing severe COVID-19 infection.
In Phase 3 clinical trials, the Sputnik V Vaccine showed strong safety, immunogenicity and efficacy results.
- During the trial, 98% of people in the vaccine group developed a humoral immune response and 100% cellular immune response.
- Vaccine recorded 91.8% efficacy for the elderly group.
- After immunization, people developed virus neutralizing antigen specific IgG antibodies that is 1.3 to 1.5 times higher than the COVID-19 recovered patients.
The Sputnik Light - Single Dose Vaccine and its efficacy
The Sputnik Light - Single Dose Vaccine based on recombinant human adenovirus serotype number 26 (rAd26), first component of the Sputnik V.
During phase 3 clinical trials, Sputnik Light vaccine reported 73.6% efficacy in preventing moderate to severe COVID-19 infection. As per Russia’s mass vaccination program between 5 December 2020 and 15 April 2021 single dose vaccine, Sputnik Light reported 79.4% efficacy in preventing symptomatic COVID-19 infection.
As per the Gamaleya National Research Center for Epidemiology and Microbiology Center, during laboratory tests the Sputnik Light showed effective results against the new variants of COVID-19. Single dose sputnik vaccine recorded 80% efficacy against the other two-dose vaccines.
How many dose and schedule of Sputnik V Vaccine?
The Sputnik V Vaccine (Gam-COVID-Vac) based on two different extremely safe human adenoviruses as vectors - Adenovirus 26 (Ad26) and Adenovirus 5 (Ad5). Human adenoviruses based medicines have been used widely for more than 50 years.
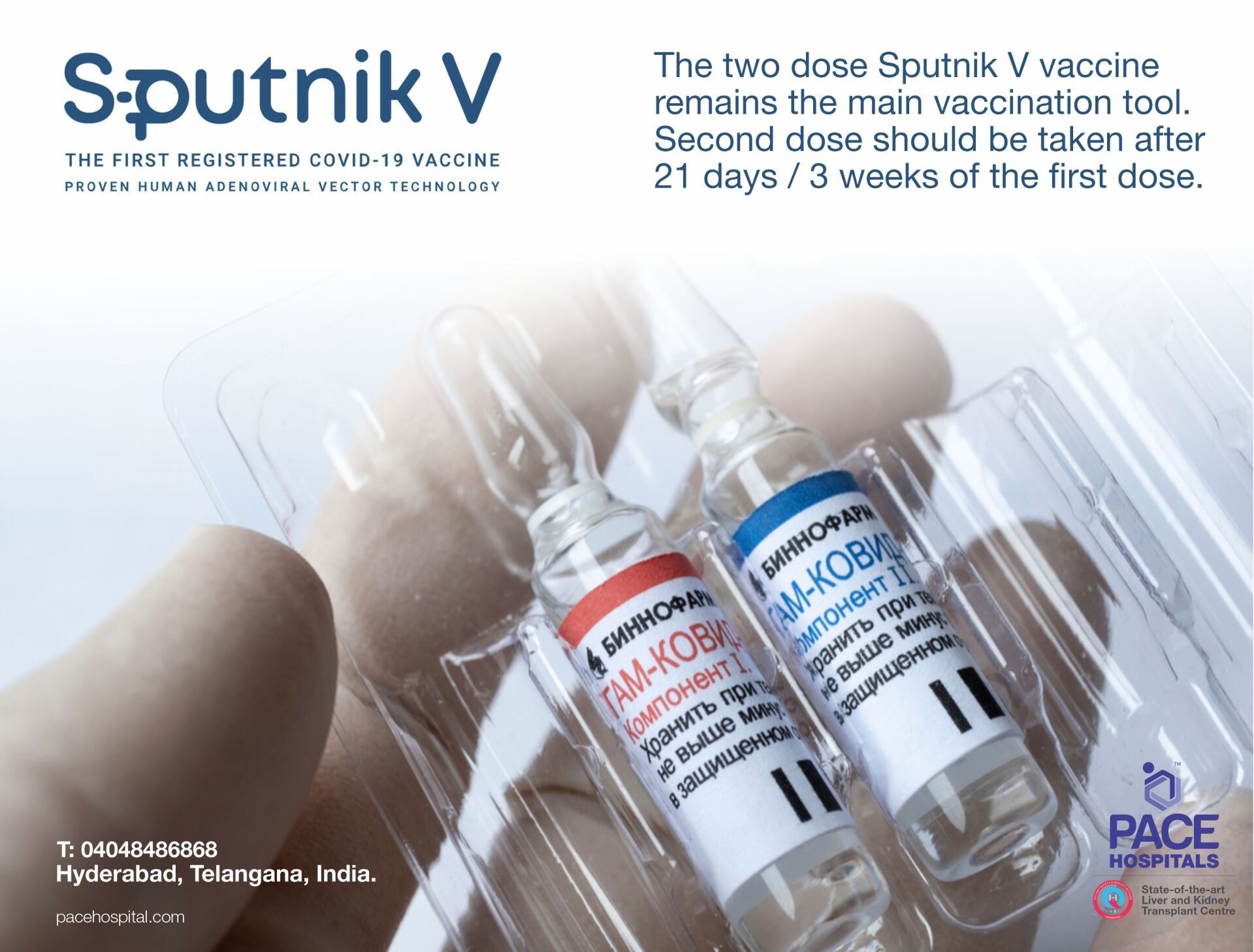
The Sputnik V - the two-dose schedule vaccine has been administered to the people who are 18 years of age and above. The second dose should be taken after 21 days / 3 weeks gap of the first dose.
What is the price / cost of Sputnik V Vaccine in India?
On 21st June 2021 under new vaccination policy Shri Narendra Damodardas Modi, Prime Minister of INDIA announced all above the age of 18 would get free vaccine. The two-dose Sputnik V vaccine currently administered in INDIA free of cost to the general population through the Centre allocated to states and union territories based on criteria such as disease burden, population and vaccination progress.
Central government will buy 75% of vaccines from the manufacturer in INDIA and distribute to states. Private hospitals can procure the rest 25% of the vaccines from the manufacturer.
As per the Government of India instructions, the private hospitals may charge up to a maximum of Rs 150 per dose as service charges per dose, respective state government will be monitoring these charges.
The maximum retails price for each dose of the Sputnik V Vaccine is Rs 995 inclusive of all taxes. First dose consists 0.5 ml rAD26 (recombinant adenovirus serotype 26 particles) and second dose consists 0.5 ml rAD5 (recombinant adenovirus serotype 5 particles).
Combining the maximum retails price and service charges at private hospitals, the vaccination price for each dose of the Sputnik V Vaccine price in India would cost Rs 1,145 inclusive of all taxes.
How to get Sputnik V Vaccine in India?
In April 2021, Drugs Controller General of India (DCGI) has been given approval for restricted emergency use of "The Sputnik V vaccine" in INDIA.
On 14 May 2021 Russia’s Sputnik-V vaccine also called as Gam-COVID-Vac, a two combined vector vaccine, has been launched in INDIA by Dr Reddy’s Laboratories Limited (DRL), Hyderabad-based pharmaceutical company.
G V Prasad, managing director of Dr Reddy’s Laboratories said - “COVID-19 vaccination is most effective tool to fight against coronavirus, everyone should come forward and take vaccine."
We Pace Hospitals have started administering the Sputnik V Vaccine at our Hitech City Branch located near Hitech City Metro Station, Hyderabad, Telangana, India and at Sarath City Capital Mall, Gachibowli - Miyapur Rd, Whitefields, Hitech City, Kondapur, Hyderabad, Telangana under #LargestVaccineDrive initiative.
Vaccine Centres will provide onsite registration facilities, people can walk-in directly to vaccination centres and get vaccinated or else book Sputnik V Vaccine slot through online CoWin registration in INDIA.
Serum Institute of India - the Sputnik V COVID-19 vaccine
The Drug Controller General of India (DCGI) gave approval to import cell and vector samples from Russia's The Gamaleya National Center of Epidemiology and Microbiology.
Kirill Dmitriev, CEO of RDIF said: "Strategic partnership with Russian Direct Investment Fund (RDIF) and Serum Institute of India (SII) will help in scaling up the production with high efficacy and excellent safety profile. Globally, this will help in battling with COVID-19 and saving lives."
Serum Institute of India Pvt. Ltd., world's largest vaccine manufacturer by number of doses produced and sold globally will start manufacturing the Sputnik V Vaccine in India, first batch will start rolling from September 2021. RDIF mentioned, around 30 crore doses will be produced in INDIA per year.
Mr. Adar Poonawalla (CEO), SII said "High efficacy and excellent safety profile, the Sputnik V Vaccine is available for the people across the globe and strengthen people to fight against SARS-CoV-2."
What are the side effects of Sputnik V Vaccine?
The Sputnik V Vaccine recorded excellent safety results and proven effective against new strains of coronavirus. 100% people showed cellular immune response against the S Protein of SARS-CoV-2.
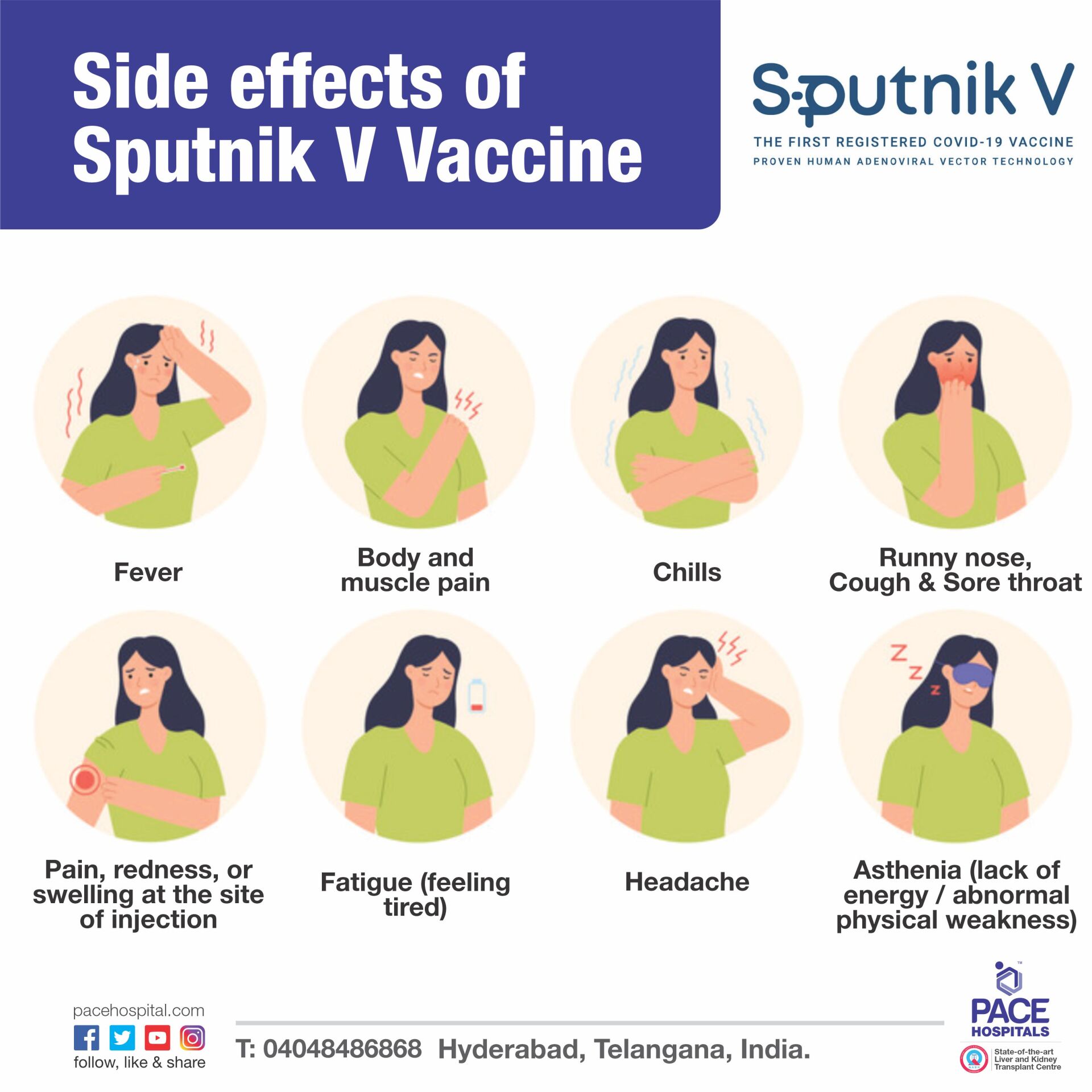
Some people reported mild side effects of Sputnik V Vaccine, list includes:
- Pain, redness, or swelling at the site of injection
- Asthenia (lack of energy / abnormal physical weakness)
- Fatigue (feeling tired)
- Body and muscle pain
- Cough and Sore throat
- Runny nose
- Fever and Chills
- Nausea and Vomiting
- Diarrhea
- Headache
Usually side effects go away within 24 to 48 hrs, if any side effects persist for longer than 48 hrs contact the vaccination centre or your physician and start medications to overcome side effects.
There was zero evidence of serious adverse events and anaphylactic shock after vaccination. Some people have not showed any side effects, everyone responds differently to the vaccine. It is normal if not having any side effects.
Share on
Request an appointment
Fill in the appointment form or call us instantly to book a confirmed appointment with our super specialist at 04048486868