IgA Nephropathy (Berger’s Disease) - Symptoms, Causes, Diagnosis, Treatment & Prevention
PACE Hospitals
Overview | Prevalence | Classification | Symptoms | Causes | Risk Factors | Pathophysiology | Complications | Diagnosis | Treatment | Prevention | FAQs | When to consult a Doctor
What is IgA nephropathy?
IgA nephropathy definition
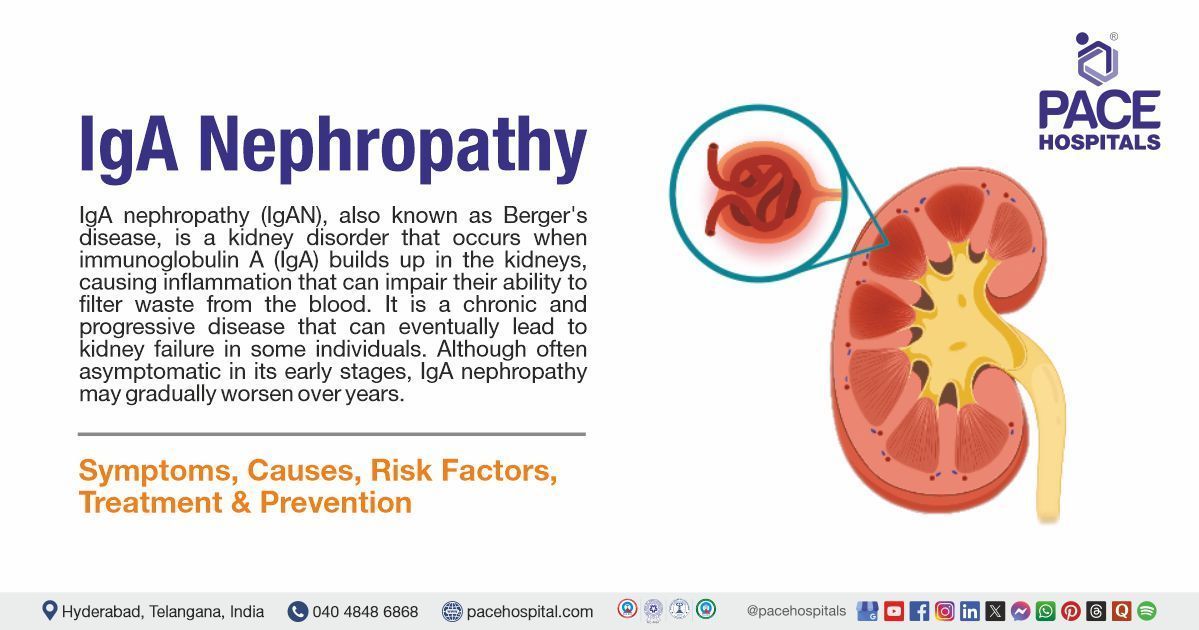
IgA nephropathy (IgAN), also known as Berger's disease, is a kidney disorder that occurs when immunoglobulin A (IgA) builds up in the kidneys, causing inflammation that can impair their ability to filter waste from the blood. It is a chronic and progressive disease that can eventually lead to kidney failure in some individuals. Although often asymptomatic in its early stages, IgA nephropathy may gradually worsen over the years.
The condition commonly begins in adolescence or early adulthood and often goes unnoticed until routine urine tests detect blood or protein. IgA nephropathy is managed by nephrologists and healthcare professionals with a focus on controlling symptoms, preventing complications, and slowing disease progression.
IgA nephropathy meaning
The term IgA nephropathy, also known as Berger’s disease, is composed of constituent terms that describe the condition’s pathophysiology and historical discovery.
IgA: This refers to Immunoglobulin A, a critical antibody in the immune system, predominantly found in mucosal regions such as the gastrointestinal and respiratory tracts. In IgA nephropathy, abnormal deposits of IgA accumulate in the glomeruli, the kidneys’ filtration units, leading to inflammation and renal impairment. The “IgA” component directly indicates this immunological mechanism.
Nephropathy: Derived from Greek, “nephros” (kidney) and “pathy” (disease, from pathos), this term denotes a pathological condition affecting the kidneys. Thus, “IgA nephropathy” precisely describes a renal disorder caused by IgA deposition.
Berger’s disease: The condition is named after Jean Berger, a French nephrologist who, alongside Nicole Hinglais, first characterized it in 1968. Their pioneering work identified IgA as the primary immunoglobulin in glomerular deposits, distinguishing this entity from other nephropathies.
IgA Nephropathy Prevalence
Prevalence of IgA nephropathy worldwide
IgA nephropathy is the most common kind of glomerulonephritis worldwide, with an estimated global incidence of 2.5 per 100,000 individuals each year. However, prevalence varies substantially by geographic region and ethnic group.
Prevalence of IgA nephropathy in India
The prevalence of IgA nephropathy in India is expected to be around 16.5%. It is regarded as the most frequent primary glomerulonephritis in India. While some studies reveal a lower prevalence of 7-16%, others report a greater prevalence of 16.5%. IgA nephropathy is more common in younger people, typically in their 20s and 30s, than in other populations.
The estimated IgA nephropathy incidence in India is reported to be between 0.2 and 5 per 100,000 individuals per year
IgA Nephropathy Classification
IgA nephropathy is generally classified into two main types: primary IgA nephropathy and secondary IgA nephropathy.
Primary IgA nephropathy is the more common form, where IgA deposits in the kidneys without a known underlying cause. Secondary IgA nephropathy, on the other hand, is associated with other medical conditions or diseases.
IgA Nephropathy Symptoms
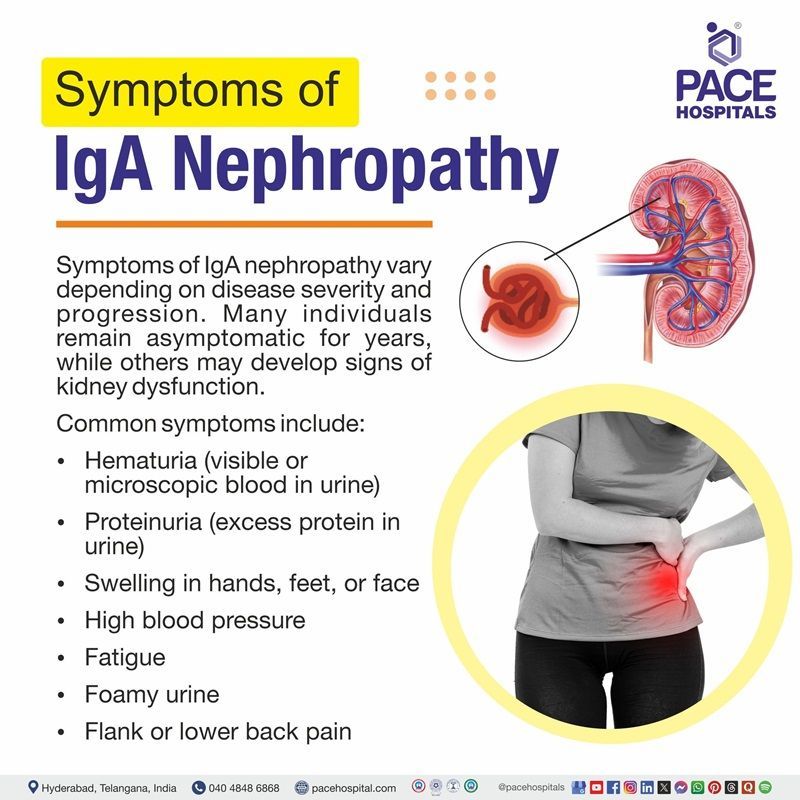
Symptoms of IgA nephropathy vary depending on disease severity and progression. Many individuals remain asymptomatic for years, while others may develop signs of kidney dysfunction.
Common symptoms include:
- Hematuria (visible or microscopic blood in urine)
- Proteinuria (excess protein in urine)
- Swelling in hands, feet, or face
- High blood pressure
- Fatigue
- Foamy urine
- Flank or lower back pain
Hematuria (blood in urine)
Hematuria is one of the most common signs of IgA nephropathy. It occurs when the glomeruli become inflamed and allows red blood cells to leak into the urine. This can result in either microscopic hematuria, detected only by urine tests, or visible hematuria, where the urine appears pink, red, or cola-colored—often triggered by infections like colds.
Proteinuria (excess protein in urine)
Proteinuria happens when damaged glomeruli lose their ability to retain proteins, allowing them—especially albumin—to pass into the urine. This leakage not only indicates kidney injury but can also contribute to worsening renal function. It may be present as foamy urine and is an important marker of disease severity.
Swelling (edema) in hands, feet, or face
Swelling, or edema, arises due to the loss of protein in the urine, which lowers the blood’s ability to hold fluid within vessels. As a result, fluid seeps into surrounding tissues, causing puffiness in areas like the hands, feet, and face. Sodium and fluid retention from impaired kidney function further aggravate this symptom.
High blood pressure (hypertension)
High blood pressure in IgA nephropathy results from disrupted kidney regulation of blood volume and hormones like renin. The kidneys may retain sodium and water, leading to increased fluid volume and vascular resistance. Uncontrolled hypertension not only reflects kidney stress but also accelerates its deterioration.
Fatigue
Fatigue in IgA nephropathy is often caused by anemia due to decreased production of erythropoietin, a hormone the kidneys produce to stimulate red blood cell formation. Additionally, the buildup of waste products in the blood (uremia) and chronic inflammation can make patients feel persistently tired and weak.
Foamy urine
Foamy urine is a visual indicator of proteinuria and occurs because protein in the urine changes its surface tension, allowing bubbles to form and persist. While occasional foam can be normal, persistent frothiness may be an early sign of significant protein leakage due to glomerular damage.
Flank or lower back pain
Some individuals with IgA nephropathy experience pain in the flank or lower back area, near the kidneys. This discomfort may result from inflammation, stretching of the kidney capsule, or episodes of gross hematuria. Although not always present, it can occur during active phases of the disease.
Other symptoms (IgA nephropathy flare up symptoms)
- Weakness and tiredness.
- Decreased urine output or little/no urination.
- Rashes, itchy or dry skin.
- Muscle cramps.
- Loss of appetite, nausea, vomiting, weight loss.
- Trouble concentrating, drowsiness, confusion.
- Headaches and sleep problems.
Many patients remain asymptomatic in the early stages, and the disease may only be discovered when blood or protein is found in the urine during routine testing. Symptoms typically worsen as kidney function declines. In severe cases, IgA nephropathy can progress to kidney failure, which is life-threatening without treatment.
IgA Nephropathy Causes
The exact cause of IgA nephropathy is unknown, but it is believed to result from an abnormal immune response. Genetic, environmental, and mucosal immune factors all play a role.
- Primary causes of IgA nephropathy include:
- Abnormal IgA1 Production
- Autoantibody Formation
- Immune Complex Deposition in the Kidneys
- Inflammatory Response and Complement Activation
- Genetic and Environmental Influences
- Other Contributing Factors
Abnormal IgA1 Production
In IgA nephropathy, the immune system produces an altered form of the immunoglobulin A1 (IgA1) molecule. Specifically, this form is deficient in galactose sugars in its hinge region. This abnormal glycosylation alters the molecular structure, making it prone to recognition by the body’s immune system as a foreign substance, which sets off an immune reaction.
Autoantibody Formation
The immune system perceives the galactose-deficient IgA1 as abnormal and responds by producing autoantibodies (usually IgG or IgA). These antibodies bind to the faulty IgA1 molecules, forming circulating immune complexes. This process indicates a breakdown in immune tolerance, which is central to autoimmune-related mechanisms.
Immune Complex Deposition in the Kidneys
The immune complexes formed between abnormal IgA1, and autoantibodies are not effectively cleared from the bloodstream. Instead, they deposit in the mesangial region of the glomeruli in the kidneys. This deposition triggers local immune responses and inflammation, as the kidney perceives these complexes as threats.
Inflammatory Response and Complement Activation
Once deposited, the immune complexes activate various components of the immune system, particularly the alternative and lectin complement pathways. This leads to the recruitment of immune cells and the release of pro-inflammatory cytokines and reactive oxygen species. These inflammatory mediators damage the glomerular structure, impairing its ability to filter blood properly and causing proteinuria and hematuria.
Genetic and Environmental Influences
Genetic factors predispose certain individuals to produce abnormal IgA1 or respond poorly to immune complex accumulation. Environmental triggers—such as infections (commonly respiratory or gastrointestinal)—stimulate mucosal immunity and increase IgA production, accelerating the disease process. Repeated infections or other environmental stresses may perpetuate this immune activation and worsen kidney injury.
Other Contributing Factors
Beyond the well-established core mechanisms, several additional causes have been identified that contribute to the development and progression of IgA nephropathy. These include:
- Impaired Clearance of Immune Complexes
- Mucosal Immune System Overactivation
- Altered Glycosylation Pathways
- Aberrant T-Cell Regulation
- Epigenetic Modifications
- Microbiome Imbalance (Dysbiosis)
IgA Nephropathy Risk Factors
IgA nephropathy is a complex kidney disorder influenced by various risk factors that affect susceptibility, onset, and disease progression. These factors contribute to the abnormal immune responses and glomerular injury characteristic of the condition. Understanding these risk elements is essential for early detection, risk stratification, and targeted management. The following are key risk factors associated with IgA nephropathy, each playing a distinct role in its pathogenesis.
- Family history and genetics
- Age
- Sex
- Ethnicity
- Associated medical conditions
- Infections
- Lifestyle and Dietary Factors
- Geographic and Environmental Factors
Family History and Genetics
Genetic predisposition plays a significant role in IgA nephropathy. Individuals with a family history of the disease have a higher risk of developing it. Specific genetic variations affect immune regulation, mucosal immunity, and the glycosylation of IgA1. These inherited traits can lead to the production of structurally abnormal IgA1 molecules, which are prone to forming immune complexes that deposit in the kidneys and cause inflammation.
Age
IgA nephropathy can occur at any age but is most commonly diagnosed in late adolescence to early adulthood, typically between 15 and 35 years. The disease onset in younger individuals is often linked to more aggressive immune responses, while older adults may experience slower progression but may also have comorbidities that complicate outcomes.
Sex
Males are more frequently affected by IgA nephropathy than females, with a male-to-female ratio of approximately 2:1 in many populations. The reasons for this disparity are not entirely understood but may be related to hormonal differences or immune system modulation, which can influence IgA production and deposition.
Ethnicity
The prevalence and severity of IgA nephropathy vary by ethnicity. It is more common in East Asian populations (e.g., Japan, China, Korea) and less frequent in African or African - American populations. These differences suggest that genetic background and possibly region-specific environmental exposures influence susceptibility and disease behavior.
Associated Medical Conditions
Certain conditions can increase the risk of developing IgA nephropathy. These include liver diseases (e.g., cirrhosis), celiac disease, and inflammatory bowel disease (IBD), all of which can stimulate abnormal immune responses and increased production of IgA. In such cases, IgA nephropathy may occur as a secondary manifestation of the underlying disease.
Infections
Upper respiratory and gastrointestinal infections often precede episodes of visible hematuria in patients with IgA nephropathy. These infections can trigger mucosal immune responses that lead to overproduction or abnormal glycosylation of IgA1. The excess or altered IgA1 forms immune complexes that deposit in the glomeruli, initiating inflammation and damage.
Lifestyle and Dietary Factors
Although not direct causes, lifestyle factors such as high sodium intake, poor diet, smoking, and obesity may exacerbate the disease by increasing blood pressure and proteinuria. Some evidence suggests that diets high in processed foods or lacking in anti-inflammatory nutrients may influence disease progression indirectly through their impact on immune health and kidney function.
Geographic and Environmental Factors
The incidence of IgA nephropathy varies geographically, with higher rates in East Asia and lower rates in North America and Africa. This variation may reflect differences in genetic predisposition, environmental exposures, diet, and healthcare practices. Certain environmental factors such as pollution or exposure to specific pathogens may influence the mucosal immune system and IgA production.
IgA Nephropathy Pathophysiology
The development of IgA nephropathy involves a complex immune-mediated process described by the widely accepted multi-hit hypothesis. The key steps of IgA nephropathy pathogenesis include:
- Overproduction of galactose-deficient IgA1 (Gd-IgA1)
- Formation of autoantibodies against Gd-IgA1
- Creation of circulating immune complexes
- Deposition of immune complexes in the glomerular mesangium
- Activation of mesangial cells and complement pathways
- Inflammation, glomerular injury, and progressive kidney damage
Overproduction of Galactose-Deficient IgA1 (Gd-IgA1)
The process begins with excessive production of IgA1 molecules that lack proper galactose residues in their hinge region. These galactose-deficient IgA1 molecules are produced mainly in the mucosal immune system (e.g., respiratory and gastrointestinal tracts) and are more prone to immune recognition.
Formation of Autoantibodies Against Gd-IgA1
The immune system generates autoantibodies (IgG or IgA) targeting the abnormal Gd-IgA1 molecules. These antibodies recognize Gd-IgA1 as a foreign antigen, triggering an autoimmune response.
Immune Complex Formation
The Gd-IgA1 and its corresponding autoantibodies form circulating immune complexes. These complexes are large and prone to deposition due to their poor clearance from the bloodstream.
Deposition in the Glomerular Mesangium
The immune complexes are deposited in the mesangial region of the glomeruli (the central part of the kidney’s filtering unit). This deposition is a hallmark of IgA nephropathy and sets off a cascade of inflammatory events.
Mesangial Cell Activation and Complement Involvement
The deposited complexes activate mesangial cells, which begin to proliferate and release pro-inflammatory cytokines, chemokines, and growth factors. Simultaneously, the complement system is activated, especially through the alternative and lectin pathways, further enhancing inflammation and tissue damage.
Inflammation and Progressive Kidney Damage
The sustained immune response causes glomerular injury, mesangial expansion, glomerulosclerosis, and eventually tubulointerstitial fibrosis. Over time, this can lead to chronic kidney disease (CKD) and potentially end-stage renal disease (ESRD) if not managed.
Complications of IgA Nephropathy
IgA nephropathy can lead to a range of clinical complications, particularly if the disease progresses or is not well-controlled. The major complications include:
- End-stage renal disease (ESRD)
- Nephrotic syndrome
- Acute kidney injury (AKI)
- Hypertension
- Cardiovascular disease
- Persistent hematuria
- Proteinuria-related malnutrition and edema
- Electrolyte imbalances
- Hyperlipidemia
- Increased risk of infections (secondary to immunosuppressive therapy)
- Anemia
- Bone mineral disorders
- Thromboembolic events
- Psychological impact and reduced quality of life
Chronic Kidney Disease (CKD)
CKD develops as a result of ongoing immune complex deposition and inflammation in the glomeruli, which leads to scarring (glomerulosclerosis) and irreversible nephron loss. As the number of functioning nephrons declines, kidney function progressively worsens.
End-Stage Renal Disease (ESRD)
ESRD is the final stage of kidney failure and occurs when the damage caused by chronic inflammation and fibrosis becomes so extensive that the kidneys can no longer perform basic filtration. This is a direct consequence of untreated or advanced CKD.
Nephrotic Syndrome
Nephrotic syndrome arises when glomerular damage becomes severe, causing large amounts of protein to leak into the urine (proteinuria). This loss of protein, especially albumin, reduces blood oncotic pressure, resulting in edema and triggers compensatory lipid production, leading to hyperlipidemia.
Acute Kidney Injury (AKI)
AKI may occur during disease flares, particularly with episodes of gross hematuria that can cause tubular obstruction by red blood cell casts or severe inflammation. The sudden decline in kidney function may lead to oliguria and rapid accumulation of toxins and fluid.
Hypertension
Damaged kidneys are less able to excrete sodium and water, leading to volume overload. Additionally, impaired regulation of the renin-angiotensin-aldosterone system (RAAS) contributes to vasoconstriction and sustained high blood pressure, which in turn worsens glomerular injury.
Cardiovascular Disease
The combination of chronic inflammation, hypertension, hyperlipidemia, and CKD increases the risk of cardiovascular complications. Reduced kidney function is a well-established risk factor for heart failure, coronary artery disease, and stroke.
Persistent Hematuria
Hematuria results from glomerular inflammation and injury that allow red blood cells to pass into the urine. In some cases, hematuria can persist for years, reflecting ongoing but often low-grade immune activity within the glomeruli.
Proteinuria-Related Malnutrition and Edema
Significant protein loss through the urine leads to hypoalbuminemia, which lowers plasma oncotic pressure and causes fluid to shift into tissues, resulting in edema. Chronic protein loss may also lead to nutritional deficiencies and weight loss over time.
Electrolyte Imbalances
Impaired kidney function disrupts the body’s ability to regulate electrolytes such as potassium, sodium, phosphate, and bicarbonate. This can lead to hyperkalemia, metabolic acidosis, and disturbances in calcium-phosphate balance, contributing to muscle weakness, cardiac issues, and bone problems.
Hyperlipidemia
As a response to low serum albumin, the liver increases the production of lipoproteins, leading to elevated cholesterol and triglyceride levels. This condition is especially common in patients with nephrotic-range proteinuria.
Increased Risk of Infections (due to immunosuppressive therapy)
Treatment with corticosteroids and other immunosuppressants suppresses immune responses, lowering the body's defenses against pathogens. This predisposes patients to bacterial, viral, and opportunistic infections.
Anemia
As kidney function declines, erythropoietin production is reduced. This hormone is essential for red blood cell production in the bone marrow, and its deficiency leads to anemia, characterized by fatigue, pallor, and reduced oxygen delivery to tissues.
Bone Mineral Disorders
CKD affects vitamin D metabolism and phosphate excretion, leading to secondary hyperparathyroidism and abnormal calcium-phosphate balance. These changes contribute to bone demineralization, pain, and increased risk of fractures—collectively known as renal osteodystrophy.
Thromboembolic Events
In nephrotic syndrome, loss of anticoagulant proteins (like antithrombin III) and increased platelet aggregation create a hypercoagulable state. This increases the risk of thrombotic events such as deep vein thrombosis (DVT), pulmonary embolism, and renal vein thrombosis.
Psychological Impact and Reduced Quality of Life
Chronic illness, the burden of lifelong monitoring or treatment, and anxiety over disease progression can contribute to depression, stress, and reduced quality of life. Fatigue and physical limitations may further impact daily functioning and social well-being.
IgA Nephropathy Diagnosis
The diagnosis of IgA nephropathy is typically initiated upon the identification of haematuria (blood in the urine) or the incidental finding of proteinuria (protein in the urine) during routine laboratory testing. To confirm the presence and assess the extent of the disease, additional diagnostic evaluations are necessary, including:
- Urine Tests
- Blood tests
- Imaging studies
- Kidney biopsy
- Additional/Specialized Tests
Urine Tests
Urinalysis is a key screening tool. It detects:
- Hematuria: Microscopic or visible blood in the urine, often intermittent.
- Proteinuria: Presence of protein in the urine, which may be mild or significant.
- Urine protein-to-creatinine ratio (uPCR) or albumin-to-creatinine ratio (uACR): Quantifies the amount of protein loss, helping to assess kidney involvement.
Blood Tests
Bloodwork helps evaluate kidney function and rule out other causes of glomerular disease.
- Serum creatinine and estimated glomerular filtration rate (eGFR): Assess overall kidney function.
- Serum IgA levels: May be elevated in some patients, but this finding is not specific or diagnostic.
- Other blood tests: Used to exclude secondary causes of kidney disease (e.g., tests for lupus, hepatitis).
Imaging Studies
Renal ultrasound is often performed to assess kidney size, rule out structural abnormalities, and exclude other causes of kidney disease.
Kidney Biopsy
Kidney biopsy is the gold standard for diagnosis. Under the microscope, the hallmark finding is the deposition of IgA in the glomerular mesangium, confirmed by immunofluorescence staining. The biopsy also helps assess the degree of chronic damage and guides prognosis and treatment.
Additional/Specialized Tests
In selected cases, tests such as the iothalamate clearance test may be used to accurately measure kidney filtration efficiency. Serologic testing may also be performed to exclude other types of glomerulonephritis.
IgA Nephropathy Treatment
The treatment of IgA nephropathy focuses on slowing the progression of kidney damage, reducing proteinuria, and managing associated risk factors such as hypertension. As there is no definitive cure, therapy is individualized based on the severity of symptoms, protein levels in the urine, and kidney function. Treatment may range from supportive care with blood pressure control and lifestyle changes to the use of immunosuppressive agents in more aggressive cases. Early and targeted intervention is essential to preserve kidney function and delay the need for kidney replacement therapy.
Main Treatment Options for IgA Nephropathy are:
- Supportive Therapy (Primary Approach)
- Immunosuppressive Therapy (For High-Risk Cases)
- Management of Complications
- Emerging and Targeted Therapies
- Personalized and Combination Approaches
- Kidney Replacement Therapy (in End-Stage Disease)
Supportive Therapy (Primary Approach)
Supportive therapy is the foundation of treatment in most patients with IgA nephropathy. It aims to minimize proteinuria, control blood pressure, and preserve kidney function.
Supportive care may include:
- Lifestyle modifications
- Blood pressure control
- Renin–angiotensin–aldosterone system inhibitors
- Sodium-glucose cotransporter 2 inhibitors
Immunosuppressive Therapy (For High-Risk Cases)
In patients with persistent proteinuria or rapidly declining kidney function despite optimal supportive care, immunosuppressive therapy may be indicated.
Immunosuppressive therapy in IgA nephropathy may include the following medications:
- Corticosteroids
- Other immunosuppressive agents
Management of Complications
IgA nephropathy can lead to a range of complications that require dedicated treatment:
- Anemia may be addressed with dietary changes or medications that stimulate red blood cell production.
- Disturbances in bone mineral metabolism require management through phosphate binders and vitamin D regulation, particularly in chronic kidney disease stages.
- Electrolyte imbalances and acidosis should be corrected to maintain internal homeostasis and reduce symptoms.
Emerging and Targeted Therapies
Emerging targeted therapies for IgA nephropathy are focused on stabilizing kidney function, reducing proteinuria and potentially reversing disease progression. These therapies include drugs like:
- Endothelin receptor antagonists
- B-cell pathway inhibitors
- Complement inhibitors
Personalized and Combination Approaches
Combining agents that reduce pathogenic antibody production with those that suppress inflammation (e.g., complement inhibition) is an emerging strategy, especially for patients at high risk of progression
Kidney Replacement Therapy (in End-Stage Disease)
When kidney function deteriorates significantly:
- Dialysis becomes necessary to perform the filtration functions of the kidneys.
- Kidney transplantation is the most definitive treatment for end-stage kidney disease and can offer good long-term outcomes, though recurrence of IgA nephropathy in the graft may occur in some cases.
Why Choose PACE Hospitals?
Expert Super Specialist Doctors
Advanced Diagnostics & Treatment
Affordable & Transparent Care
24x7 Emergency & ICU Support
IgA Nephropathy Prevention
Preventing the onset or progression of IgA nephropathy is a crucial goal in kidney health, especially because the disease can silently lead to significant kidney damage over time. While there is currently no definitive way to prevent the initial development of IgA nephropathy due to its complex and partially unknown causes, several strategies have been identified to reduce risk factors and delay disease progression in those already affected. Prevention in this context focuses on minimizing kidney inflammation, controlling contributing conditions, and preserving long-term renal function. Early and sustained implementation of these preventive measures can make a significant difference in outcomes.
Preventive Strategies for IgA Nephropathy include:
- Maintaining Healthy Blood Pressure
- Controlling Proteinuria
- Adopting a Kidney-Friendly Diet
- Avoiding Smoking and Excessive Alcohol
- Minimizing the Use of Nephrotoxic Medications
- Managing Infections Promptly
- Regular Monitoring and Medical Follow-Up
- Exploring Emerging Therapies with Medical Supervision
Maintaining Healthy Blood Pressure
Controlling blood pressure is one of the most effective ways to slow the progression of IgA nephropathy. High blood pressure increases the pressure inside the glomeruli, leading to further damage and protein leakage. Maintaining a target blood pressure below 130/80 mmHg using medications like ACE inhibitors or ARBs not only helps manage hypertension but also has kidney-protective effects by reducing proteinuria and glomerular inflammation.
Controlling Proteinuria
Proteinuria, or excess protein in the urine, is a key marker of kidney damage and a predictor of disease progression. Reducing proteinuria through medications (especially ACE inhibitors or ARBs) and dietary adjustments helps decrease glomerular stress and inflammation. Keeping protein excretion below 1 gram per day significantly lowers the risk of kidney function decline.
Adopting a Kidney-Friendly Diet
Diet plays a central role in managing kidney health. A low-sodium, low-fat, and possibly low-protein diet can reduce blood pressure and kidney workload. Patients should focus on fresh fruits, vegetables, whole grains, and lean proteins while avoiding processed foods. A renal dietitian can tailor dietary plans to individual needs, especially in advanced stages of the disease.
Avoiding Smoking and Excessive Alcohol
Smoking accelerates kidney damage by promoting oxidative stress and vascular injury. It also worsens hypertension and proteinuria. Alcohol, when consumed excessively, can impair liver function and exacerbate immune system disturbances linked to IgA nephropathy. Quitting smoking and moderating alcohol intake can significantly benefit overall and renal health.
Minimizing Use of Nephrotoxic Medications
Drugs like nonsteroidal anti-inflammatory drugs (NSAIDs), certain antibiotics, and contrast agents can cause or worsen kidney injury. People with or at risk of IgA nephropathy should avoid these medications unless absolutely necessary, and only under medical supervision. Alternative pain management strategies and cautious medication review are essential.
Managing Infections Promptly
IgA nephropathy is often triggered or worsened by infections, particularly of the upper respiratory and gastrointestinal tracts. Prompt treatment of infections, maintaining good hygiene, and staying up to date with vaccinations (such as the flu shot) can help reduce immune system activation and subsequent IgA overproduction.
Regular Monitoring and Medical Follow-Up
Routine monitoring of blood pressure, kidney function (creatinine and eGFR), and urine protein levels allows early detection of disease progression. Regular follow-up with a nephrologist ensures timely intervention, medication adjustments, and the management of complications, all of which are crucial for long-term kidney preservation.
Exploring Emerging Therapies with Medical Supervision
Recent advances in nephrology are exploring drugs like SGLT2 inhibitors, endothelin receptor antagonists, and complement pathway inhibitors. While still under research or in early clinical use, these therapies may offer hope for better outcomes in IgA nephropathy. Patients should discuss clinical trial options or novel treatments with their healthcare provider.
Frequently Asked Questions (FAQs) on IgA Nephropathy
Can IgA nephropathy be reversed?
IgA nephropathy can show significant reversibility, particularly when treatment is started early. Studies have demonstrated that mesangial proliferation and interstitial changes can regress, and a histological cure is possible in a considerable proportion of patients, especially if intervention occurs at an early stage. However, complete reversal is rare in adults, and long-standing damage may not be fully reversible.
Can IgA nephropathy cause death?
IgA nephropathy can lead to chronic kidney disease and, in some cases, progress to end-stage kidney disease (ESKD), which can be fatal if not managed with dialysis or kidney transplantation. Mortality is associated with severe disease, high proteinuria, and declining kidney function.
Is IgA nephropathy hereditary?
IgA nephropathy can have a hereditary component, as familial clustering has been observed. However, it is not strictly inherited in a simple Mendelian pattern. Both genetic and environmental factors contribute to the development of the disease.
What causes IgA nephropathy?
IgA nephropathy is caused by the deposition of abnormal IgA antibodies in the kidney glomeruli. These antibodies exhibit abnormal glycosylation, making them targets for other antibodies, leading to the formation of immune complexes, inflammation, and kidney damage. The exact trigger is unknown, but genetic predisposition and environmental factors play a role.
Is IgA nephropathy an autoimmune disease?
Yes, IgA nephropathy is classified as an autoimmune disease. It occurs when the immune system produces abnormal IgA antibodies, which the body recognizes as foreign. This triggers the formation of immune complexes that are deposited in the kidneys, causing inflammation and damage to the glomeruli.
Can IgA nephropathy go into remission?
IgA nephropathy can go into remission, particularly in patients with mild disease. Studies have observed relatively high rates of spontaneous remission of both hematuria and proteinuria, especially within the first three years after diagnosis. Remission is more likely in patients with lower levels of proteinuria at diagnosis.
How common is IgA nephropathy?
IgA nephropathy is the most common primary glomerular disease worldwide, but its frequency varies by region. It is more frequent in Asian populations (e.g., 45 cases per million per year in Japan) than in Caucasian populations (e.g., 31 cases per million per year in France). Differences in screening and biopsy practices can affect reported prevalence.
Can IgA nephropathy be cured?
There is currently no established permanent cure for IgA nephropathy. Treatments aim to slow disease progression, reduce proteinuria, and manage symptoms. Some patients may achieve remission or significant improvement, but most require long-term management to prevent progression to kidney failure.
How fast does IgA nephropathy progress?
The progression of IgA nephropathy varies widely. Some patients remain stable for years, while others experience rapid declines in kidney function. Recent studies show that high rates of kidney outcomes, including ≥50% decline in kidney function, kidney failure, or death, can occur within a median of 2.7 years, especially in those with higher proteinuria at baseline.
Can an IgA nephropathy patient go for kidney transplant?
Yes, patients with IgA nephropathy who progress to end-stage kidney disease are eligible for kidney transplantation. However, IgA nephropathy can recur in the transplanted kidney, though recurrence does not always lead to graft failure.
Does IgA nephropathy affect both kidneys?
IgA nephropathy typically affects both kidneys, as it is a systemic disease involving immune complex deposition in the glomeruli of both organs.
What is the IgA nephropathy life expectancy?
Research studies indicate that life expectancy in IgA nephropathy varies, but is generally modestly reduced compared to the general population. Most patients are diagnosed before age 40, and in countries where the disease is common, life expectancy ranges from 70 to 85 years. A large cohort study found that the mean age at kidney failure or death was 48 years, with most patients progressing to kidney failure within 10–15 years of diagnosis if proteinuria and kidney function decline are not well controlled.
Survival rates at 10, 20, and 30 years were 96.3%, 91.8%, and 82.7%, respectively, in one long-term study. The average lifespan is estimated to be about 6 years shorter for people with IgA nephropathy compared to the general population, especially if kidney failure develops.
What is the recommended IgA nephropathy diet?
Research does not specify a unique diet for IgA nephropathy, but recommendations focus on general kidney-protective dietary principles. These include:
- Limiting sodium intake to control blood pressure and reduce proteinuria.
- Limiting protein intake to reduce kidney workload, particularly in advanced stages.
- Managing potassium and phosphorus intake if kidney function declines.
- Emphasizing a balanced diet rich in fruits, vegetables, and whole grains, while avoiding processed foods high in salt and additives.
What are the recent advances in IgA nephropathy treatment?
Recent advances in IgA nephropathy treatment focus on targeted therapies that address specific disease mechanisms. These include agents that modulate mucosal immunity, inhibit the alternative complement pathway, and block endothelin receptors.
Clinical trials have demonstrated that such therapies can significantly reduce proteinuria and slow kidney function decline in patients with persistent proteinuria despite standard care. These developments represent a shift towards personalized, mechanism-based treatments for IgA nephropathy.
What is the IgA nephropathy prognosis?
IgA nephropathy prognosis varies widely based on disease severity, early detection, and treatment response. Approximately 20–40% of patients progress to end-stage kidney disease (ESKD) within 20 years of diagnosis. Key risk factors for poor prognosis include persistent proteinuria (>1 g/day), hypertension, impaired kidney function at presentation, and specific histological findings. Early diagnosis, blood pressure control, and proteinuria reduction are critical for improving outcomes. Regular monitoring and individualized treatment can help slow disease progression and preserve kidney function.
What is IgA nephropathy life expectancy in India?
In India, IgA nephropathy is a condition that can significantly impact life expectancy. Studies suggest a 6-year reduction in average life expectancy for individuals with IgA nephropathy, compared to the general population. Additionally, a significant portion of IgA nephropathy patients in India experience a rapid decline in renal function and may progress to end-stage renal disease (ESRD) within a shorter timeframe than seen in other populations.
When to consult a doctor for IgA nephropathy?
Consult a doctor for IgA nephropathy when symptoms like blood in the urine, foamy urine, or swelling continue for weeks or begin interfering with everyday activities. This condition occurs when IgA proteins collect in the kidneys and trigger inflammation, which, over time, can harm kidney function if not appropriately managed.
Warning signs that need medical attention include:
- Blood in urine that appears pink, brown, or cola colored
- Foamy urine linked to protein leakage
- Swelling of the ankles, legs, hands, or face
- High blood pressure that is hard to control
- Tiredness or other signs of reduced kidney function
If these problems persist or worsen, an IgA nephropathy specialist can run the proper tests and suggest effective IgA nephropathy treatment options.
Share on
Request an appointment
Fill in the appointment form or call us instantly to book a confirmed appointment with our super specialist at 04048486868